Compound Pharmacies
the burgeoning infrastructure behind personalized medicine
Good morning,
Today’s newsletter: the rise of compound pharmacies
Most healthcare innovation in providing patient care has been an attempt to skirt regulatory capture in order to deliver cheaper and better patient outcomes.
From a technology perspective, this has led to the rise of telehealth providers, who mainly function as prescription writers that are compliant and fast.
From a capital perspective, it has led to the rise of MSO structures to arbitrage regulations around owning medical practices.
But perhaps the most important trends here are consumer-related and drug related. There’s a large push towards self-determination, proactive solutions, and personalized care.
The easiest way to see this is in pharmaceutical data:
TRT therapy grew from 7.3 million in 2019 to more than 11 million in 2024.1
GLP-1 agonists like Ozempic are projected to reach $114B in domestic spend over the next 7 years.
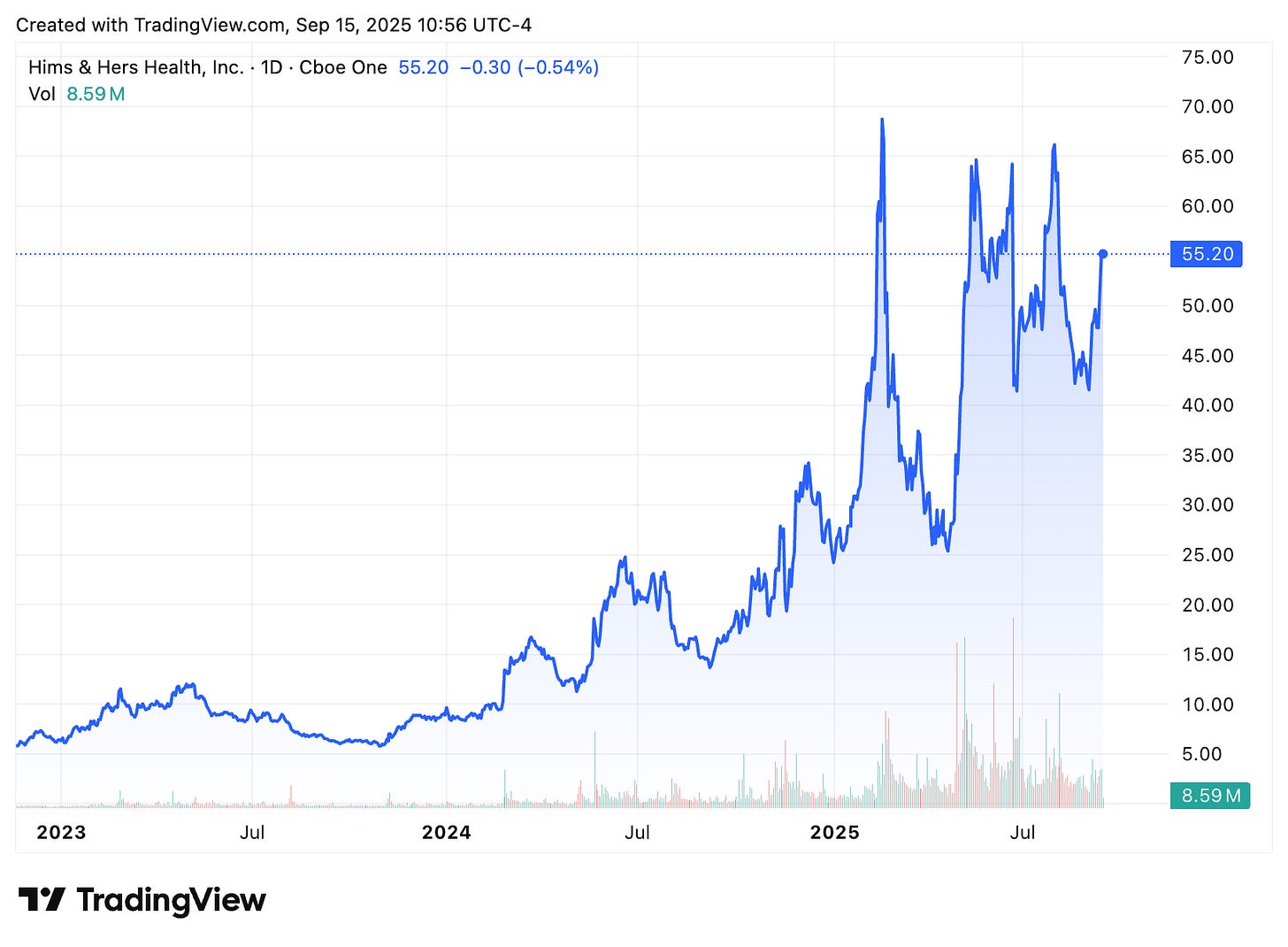
Or, we can just look at HIMS 0.00%↑, which is now up 233% over the past year, primarily attributable to their ability to write personalized prescriptions including weight loss drugs.
Hims also happens to be a fantastic place to start to analyze the market segment we are interested in today: the compound pharmacy market.
Notably, HIMS had a deal with Novo Nordisk to prescribe WeGovy en masse. Novo Nordisk provides the drug, HIMS provides the market. That deal collapsed in June due to accusations that HIMS was engaged in an “illegal compounding operation”.
How does one engage in an illegal compounding operation you ask? The short answer: there’s nothing truly illegal about it.2 Because there’s one other large trend in healthcare: compound pharmacies are becoming indispensable through regulatory arbitrage for this alternative healthcare system that is forming.
Compounds vs. Generics
Let’s start with clearing something up: Compound drugs - what we are talking about - are different from generic drugs.
A generic drug is simply a version of a drug whose patent has lapsed. The FDA approves these and regulates their manufacturing. The drugs themselves are mass-manufactured.
Compound drugs are markedly different. Historically, there are a number of reasons these cropped up. Every healthcare system needs two important things:
Surge capacity: If there is a market shortage of a drug, legal frameworks for legally creating these drugs without patent concerns are necessary for patient safety and national security.
Personalization: Since drugs are now primarily mass manufactured, there’s a certain set of doctors and patients that need different dosages, formulation considerations, and more.
And as a result, the US as a matter of history and more recently as a matter of law, has authorized compounding pharmacies to operate.
The important features here are as follows:
Compound pharmacies must utilize FDA-approved ingredients but the drugs they compound are not subject to FDA regulation.
Compound pharmacies must not make “copies” of patent-protected drugs… unless there’s some exceptional circumstance requiring surge capacity.
I’m sure you can quickly see where this is going. All the action in compounding pharmacies is concentrated on a) what constitutes a copy, and b) what constitutes exceptional circumstances.
This is best seen in the Ozempic frenzy that has occurred. Initially, the demand for weight loss medication was greater than the drug companies could manage. As a result, the FDA essentially allowed compound pharmacies with no questions asked to compound weight loss medication.3 As the shortage has ended, a large part of the telehealth ecosystem, patients, and physicians are not thrilled to go back to the drug manufacturers.
And so now the other aspect of the question is being litigated:
Are pharmacies that compound the peptides underlying Wegovy and Ozempic “copying” a patented drug?
Simply put: the number of peptide-based therapies, hormonal therapies, and certainly other currently hidden personalized treatments are only set to grow. This standoff between drug manufacturers and pharmacies will continue.
But there’s a couple of reasons, the compounding pharmacy market will continue to grow:
It’s too easy to get the ingredients from Chinese manufacturers and do DIY compound. This is a big liability for the FDA due to low visibility and similar national security reasons. If you overregulate and shut down compound pharmacy operations, risks to patient safety grow.
MAHA is in the White House: In the near term, there’s no federal support for shutting down the compound pharmacy market, in fact it seems likely to grow.
Personalized medication grows and will continue to ensure these drugs are subject to a large carveout. The imagined punishment behind the FDA’s “don’t copy the drug manufacturers products” regulations hinges upon an statute:
The statute… states that “the term ‘essentially a copy of a commercially available drug product’ does not include a drug product in which there is a change, made for an identified individual patient, which produces for that patient a significant difference, as determined by the prescribing practitioner, between the compounded drug and the comparable commercially available drug.”4 (emphasis added)
And herein lies the enduring regulatory arbitrage: as personalized medication grows due to unique patient data and physician knowledge (and let’s be honest due to wanting to exercise this arbitrage), the alternative healthcare system is incentivized in the interest of its patients to procure “boutique” medication that fits the exact needs of the patient herself.
And therein you have the entire case for the compounding pharmacy boom over the next 10-15 years.
Takeaways:
From an investor or builder perspective, there’s numerous pathways to creating value in this segment of the market.
Many compounding pharmacies are boutique operations run by small teams.5 They look like SMBs. It is easy to imagine software solutions that manage ingredient procurement and inventory, prescription management. What if you built aspects of Slice but for compounding pharmacies?
But you can also imagine this from a franchise perspective: since many of these are boutique operations, there are likely benefits from centralizing aspects of the operation whether physician awareness, marketing, and procurement. Does the next CVS look like a network of compounding pharmacies?
And lastly, in the 503b segment, as more hospital systems, ASCS, and others come around to compounding more of their prescriptions, the value of these facilities will grow substantially leading to buyouts, and many other potential business models.
Right “Rough estimates are that compounded medications account for 1% to 3% of all prescriptions written in the U.S.”6 What happens when that number goes to 3-5%? Or as personalized medication grows to 10%?7
In short, I think compounding pharmacies are one of the more interesting markets to innovate around these days and I’m very interested in hearing from founders and operators building in this space.
We will be back Wednesday. Thanks again for being a supporter of Verticalized.
Not legal advice, not financial advice.
This is true in the 503a segment and not necessarily the 503b segment, but this piece would be too long to dive into this fully.
Someone likely has a great model around the domestic carrying capacity for compounded medication. This is an approximation. It would be highly interesting to look at an estimate around how many medications could realistically be compounded.